The year 2020 has carved its mark in the history of the world. After waiting for a very long time, India is set to begin its first Vaccination drive, with its first phase commencing from 16th January 2021. There are various vaccines amidst the pandemic in the global market like Pfizer, Moderna that are been used for immunization against Coronavirus. India produced two vaccines Covishield and Covaxin, Covishield has been tested and has proven its efficacy.
Statistically speaking, Jaipur has the highest number of active cases with 58,512 cases, following which is Jodhpur with 44,574 active cases. Udaipur has 11,691 active cases, which after the vaccination drive should drop further.
Udaipur’s Vaccination drive
- In Mid-January Udaipur received its first phase of COVID-19 vaccination, COVISHIELD ahead of the mass vaccination drive starting on 16th January 2020.
- The Government of India has prioritized the health workers and the frontline workers for the vaccination, following which the vaccine would be administered to high-risk people and the elderly population.
- Udaipur will have 9 centers for the planned vaccinations as per the city government.
- There will be three sections to every center: Waiting area, Vaccination Area, Under observation area. The recipients of the vaccination will be observed for 30 minutes after the vaccination to check for any side-effects from the vaccine.
- Recipients who want to get the vaccination have to register on the COWIN app as directed by the Government of Rajasthan and the city of Udaipur.
- For emergencies related to Coronavirus, anyone can call the numbers 104 and 108 for immediate assistance.
What are the vaccines for Coronavirus?

India has produced two vaccines to fight Coronavirus, COVISHIELD, and COVAXIN. Covishield is developed by the Serum Institute of India, while Covaxin is developed by Bharat Biotech., a Biotechnology Company headquartered in Hyderabad.
COVISHIELD
Covishield is a COVID-19 vaccine developed by Serum Institute of India, headquartered in Pune. The vaccine has been approved by DCGI and is under circulation for mass vaccination drives planned ahead this year by the government of India. Covishield is the only vaccine produced by India that has proven efficacy. The vaccine is given to some states while the other states have received Covaxin. The vaccines are not interchangeable.
Udaipur has received 1,00,500 Covishield vaccines last week for the first phase of the vaccination drive.
Who should not take the vaccine?
- People who are exhibiting active symptoms of SARS-CoV-2 infection.
- COVID-19 patients who have been given anti-SARS-Cov-2 monoclonal antibodies or convalescent plasma.
- Critically unwell and hospitalized patients (with or without intensive care) due to any illness.
- People with a history of platelet disorder, clotting factor deficiency, or coagulopathy should only be given the vaccine after cautioning them.
Can those with certain health conditions be administered the vaccine?
- People with a past history of COVID-19 infection can be immunized.
- People with a history of chronic diseases – cardiac, neurological, pulmonary, metabolic, and malignancies – are also included.
- Patients on immunosuppression and immunodeficiency or HIV can be administered the COVID-19 vaccine.
What are the side-effects?
For Covishield, there are a few recorded mild adverse effects that may or may not happen following immunization:
- Injection site tenderness;
- injection site pain; headache;
- fatigue; myalgia (muscle pain); discomfort;
- pyrexia (an abnormal elevation of body temperature); chills; and nausea.
- In such cases, paracetamol can be given, the advisory adds.
- Very rare events of demyelinating disorders” have been reported following the vaccination, although not too many recurring cases.
- In any case of adverse effects, please contact the nearest available hospital and get examined.
COVAXIN
Covaxin is considered India’s first Indigenous COVID-19 Vaccine developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). The vaccine received DCGI approval for Phase I & II of Human Clinical Trials and the trials started across India from July 2020. The vaccine’s efficacy is not yet proven as opposed to Covishield which is proven to have worked against the virus. Before giving anyone Covaxin, they should be informed about the same. It is called mandatory Informed consent, wherein the patient can take a day to think if he wants to be immunized.
Who should take the vaccine?
- The vaccine should only be administered by the health professionals and frontline health workers who are a part of the mass drive.
- The vaccine is strictly for the patients affected by Coronavirus, health professionals, and elderly people as per the Government’s prioritization for the drive.
- The vaccine should only be taken by patients infected with the virus, in this case after the approval of the patient or their family about the unproven efficacy.
- Pregnant women should not take the vaccination
- The vaccine is only for people of 18 years and above.
What are the side effects?
The vaccine has only been approved by the Government in the Clinical Trial Mode, which would mean that the recipients would have to sign a consent form. In any events of severe or any side effects, Bharat Biotech would have to compensate for the same.
According to the Centre’s fact sheet, these are the common adverse events after administering Covaxin.
- Injection site pain
- Fever and Headache
- Body ache and Abdominal pain
- Nausea and Fatigue
- Dizziness and Sweating
- Cold and Cough
If any of these symptoms surface, post-vaccination, please contact the authorities for further examination and prevention.
Covid-19 has swept over millions of lives globally through the year 2020 and now 2021, the development of vaccines should lower the risk of the virus by a considerable amount. Hospitals and health workers are at the frontline of this crisis, be sure to thank them for their service, as we get ahead of this virus.
About Chaudhary Hospital

Established in 2005, Chaudhary Hospital in Udaipur is the city’s premier Multispecialty hospital situated in the center of the city. Our Hospital is
- NABH Certified
- 15 years of experience in Healthcare services
- 4 Lakh+ tests per year
- Team of Expert Doctors and Specialists
- Accurate Results
- Advanced Diagnostic Labs
- COVID Care unit
Our team of doctors and the entire staff of Chaudhary Hospital have been vaccinated with the Covishield vaccine. We are proud to have serve Udaipur with the utmost care and safety required to fight the pandemic.
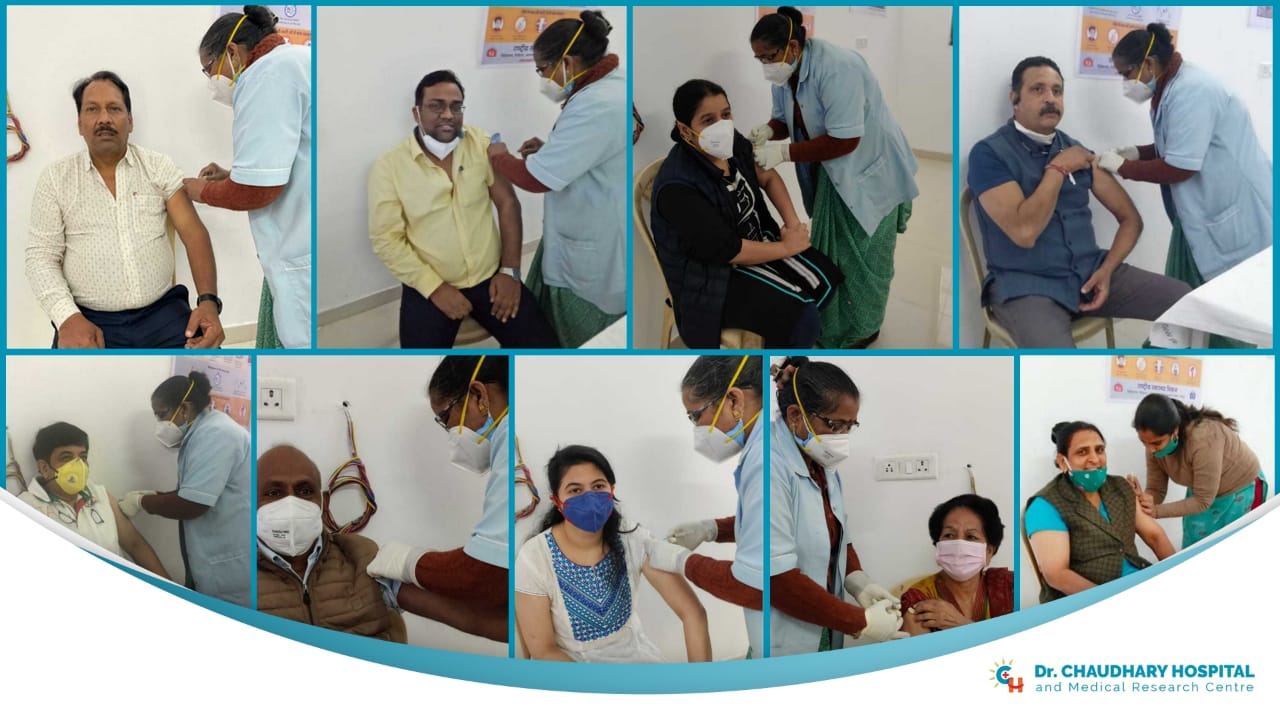
Our COVID Care unit is equipped with the latest technology and procedures. According to the guidelines by the Ministry of Health and Family Welfare, Government of India, Home Isolation is advisable for entirely asymptomatic or patients with mild symptoms. We at Chaudhary Hospital provide specialized care for COVID-19 infections. To know more, contact us on +91 294 2465566